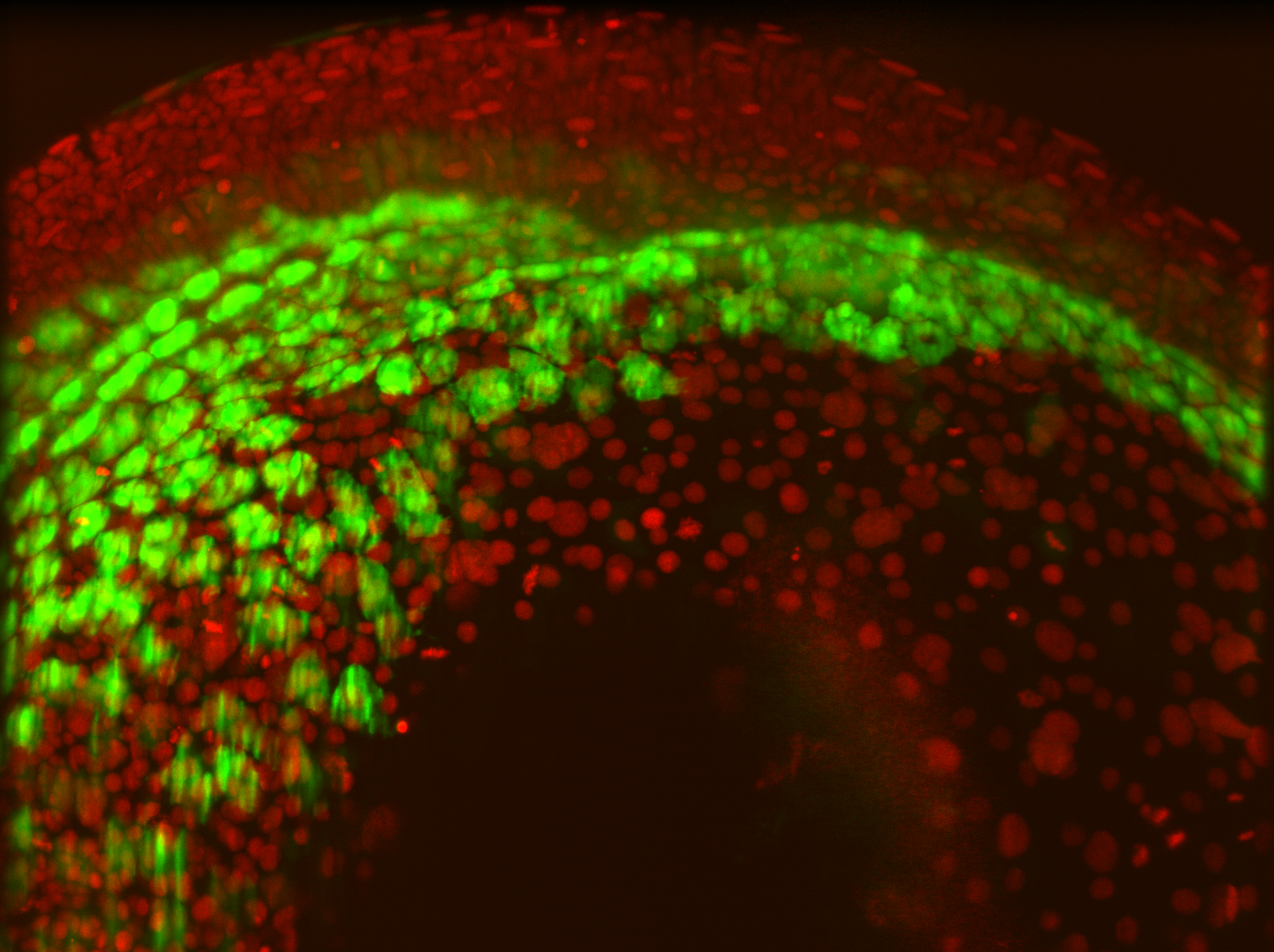
The gut epithelium is derived from the endoderm. In zebrafish, endodermal cells initially undergo highly dynamic single-cell migration before switching their migratory behavior to adhere together into an endodermal sheet. Our lab is interested in understanding how this behavioral switch is regulated at the molecular level and developing new tools to study in vivo cell biology.
How is cell-cell adhesion initiated?
Among epithelial cells, adhesion is mediated by cadherin-dependent adherens junctions (AJ). We are using whole embryo microscopy to image fluorescently labeled AJ components to systematically define how cells initiate AJ formation as they transition from single-cell migration to sheet formation
How are cell-cell interactions changing over time?
Preliminary experiments suggest that when endodermal cells are undergoing single-cell migration, they actively avoid each other by contact-mediated repulsion. However, as they transition to sheet formation, contacting membranes adhere to each other rather than repel away and yet, we observe that membrane protrusion still preferentially occurs at contact-free edges, suggesting that contact repulsion remains partially active. We want to understand how contact repulsion is regulated in the endoderm and how this behavior contributes to subsequent gut development. In particular want to identify the receptors endodermal cells use to recognize and repel away from each other, elucidate the signaling pathways that restrict membrane protrusion to cell-free edges, and understand how inhibiting contact repulsion affects gut development.

How does membrane blebbing contribute to endodermal cell motility and adhesion?
In preliminary experiments, we found that when endodermal cells are undergoing single-cell migration, they exhibit frequent membrane blebbing. Blebbing occurs when attachments between the plasma membrane and the underlying cortical actin cytoskeleton weaken or fail, allowing hydrostatic pressure from the cytoplasm to expand the membrane outward. Membrane blebbing was first characterized as a hallmark of apoptotic cells, but more recently it’s been appreciated that cells can use blebs as a mode of cell migration. We are interested in understanding how blebbing contributes to endodermal cell motility, how bleb formation is regulated in these cells, and why blebbing stops as cell transition to sheet formation.

Optogenetic control of gene expression in zebrafish
One challenge of studying organ morphogenesis is that many cytoskeletal and adhesion-related genes have broad expression patterns, making it technically difficult to restrict perturbation of these genes only to the tissue of interest. We recently developed a light-gated inducible expression system for zebrafish that enables precise, user-defined control of gene expression. This system consists of a blue-light sensitive transcriptional activator called TAEL that activates transcription downstream of the TAEL-responsive C120 promoter. We demonstrated that the TAEL/C120 system can be used with multiple imaging systems to deliver patterned blue light illumination, allowing for precise spatial and temporal regulation of gene expression. We also showed that TAEL/C120 can be used to express Cas9 nuclease to achieve inducible gene editing. Current projects in the lab are aimed at improving the TAEL/C120 system in zebrafish.